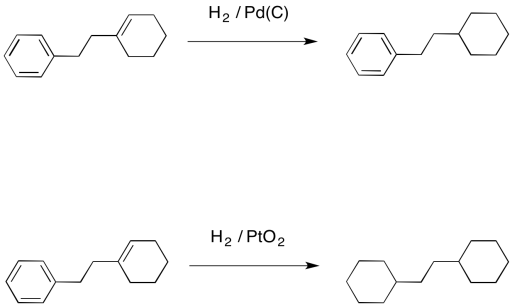
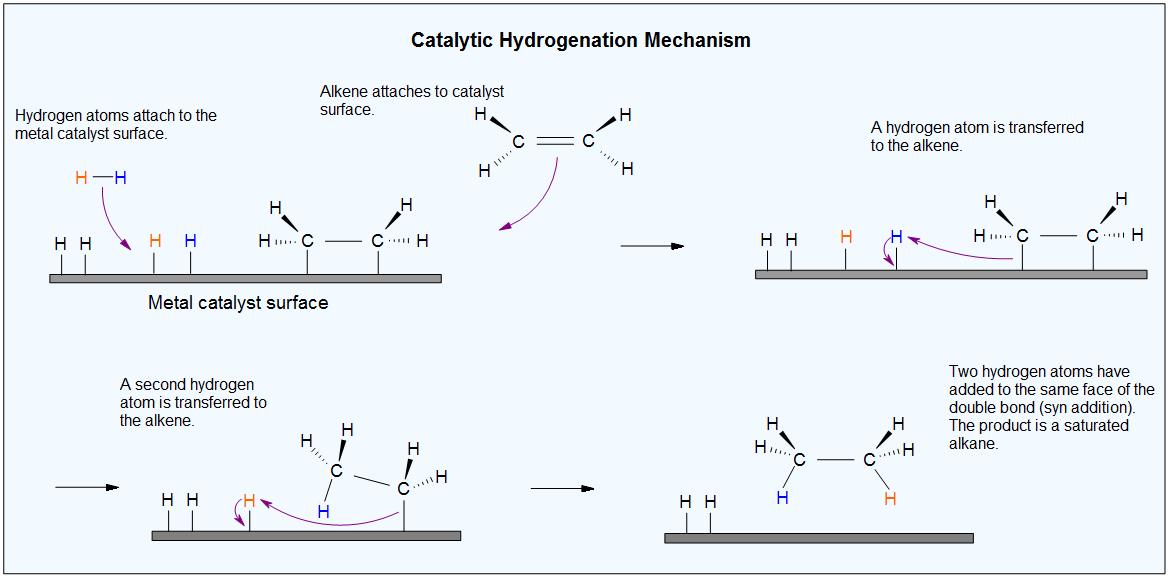
Catalysts | Free Full-Text | Hydrogen Transfer Reactions of Carbonyls, Alkynes, and Alkenes with Noble Metals in the Presence of Alcohols/Ethers and Amines as Hydrogen Donors | HTML

Water as a hydrogen source in palladium-catalyzed reduction and reductive amination of nitroarenes mediated by diboronic acid - ScienceDirect
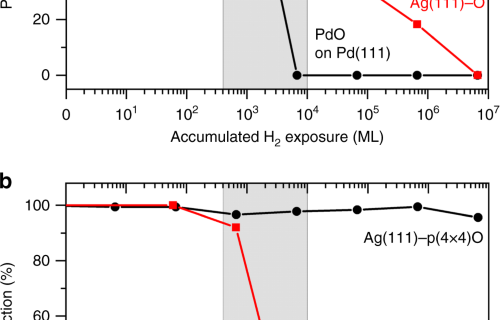
Hydrogen migration at restructuring palladium–silver oxide boundaries dramatically enhances reduction rate of silver oxide | Nature Communications

Hydrogen in Palladium and Storage Properties of Related Nanomaterials: Size, Shape, Alloying, and Metal‐Organic Framework Coating Effects - Dekura - 2019 - ChemPhysChem - Wiley Online Library

EDS spectra of (a) palladium, (b) platinum, (c) nickel, and (d) tin... | Download Scientific Diagram

Palladium nanoparticles grown on β-Mo2C nanotubes as dual functional electrocatalysts for both oxygen reduction reaction and hydrogen evolution reaction - ScienceDirect

Hydrosols of Pd and Pd-H2: Influence of particle nature on the rate of catalytic reduction of hexacyanoferrate(III) ions with hydrogen - ScienceDirect

Water as a hydrogen source in palladium-catalyzed reduction and reductive amination of nitroarenes mediated by diboronic acid - ScienceDirect

An Efficient, Stable and Reusable Palladium Nanocatalyst: Chemoselective Reduction of Aldehydes with Molecular Hydrogen in Water - Kotha - 2016 - Advanced Synthesis & Catalysis - Wiley Online Library

PDF) Pd/P( t -Bu) 3 : A Mild Catalyst for Selective Reduction of Alkenes under Transfer-Hydrogenation Conditions
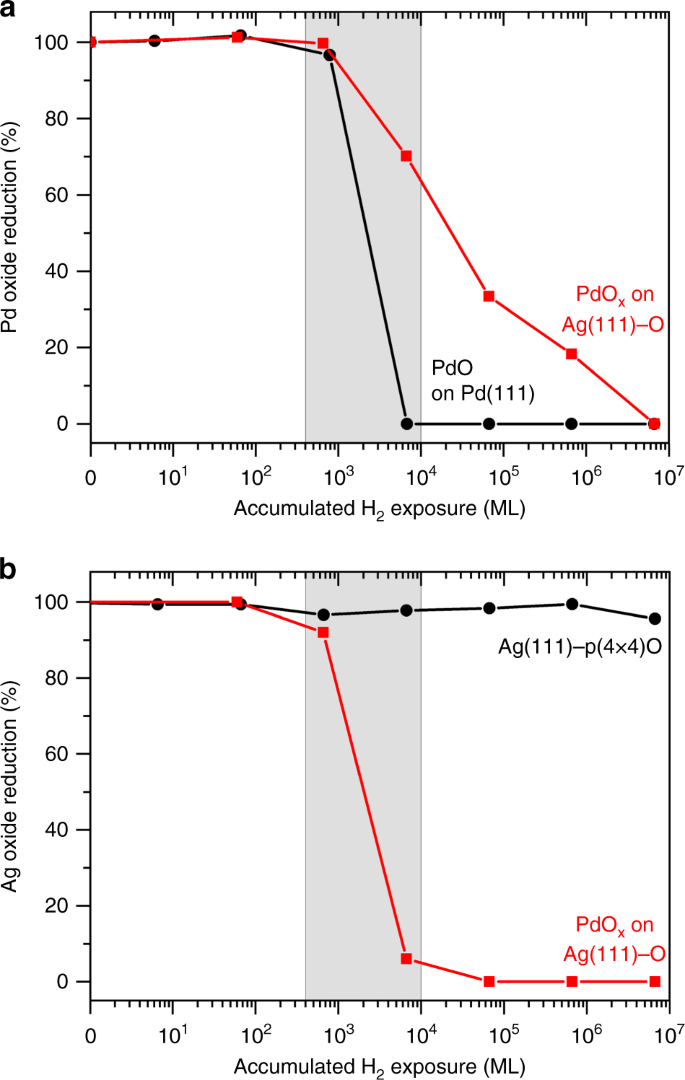
Hydrogen migration at restructuring palladium–silver oxide boundaries dramatically enhances reduction rate of silver oxide | Nature Communications

Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole - ScienceDirect
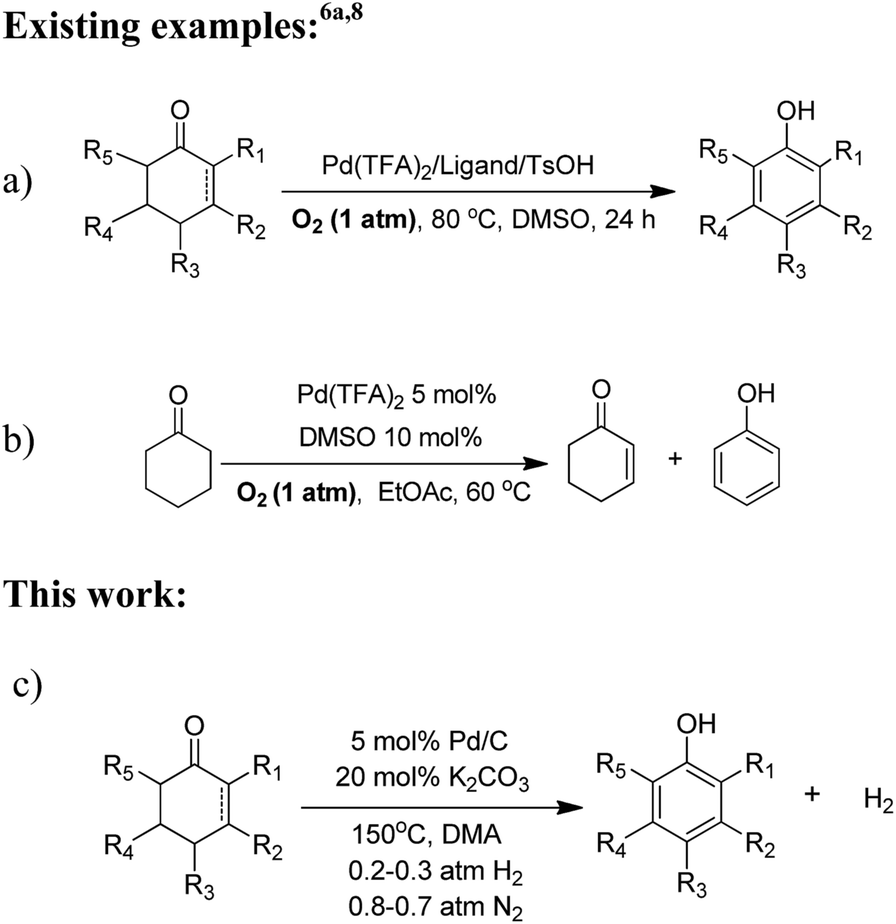
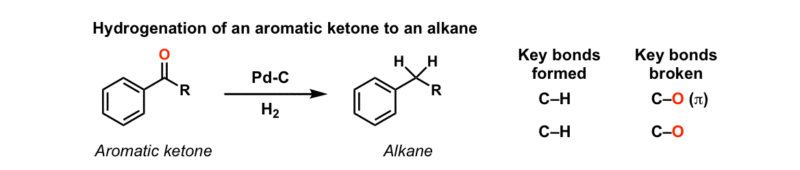

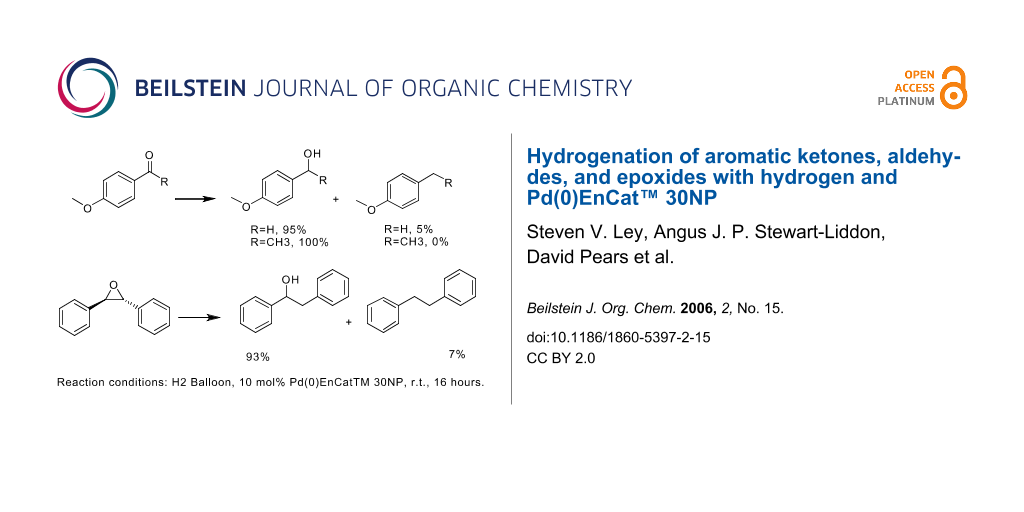

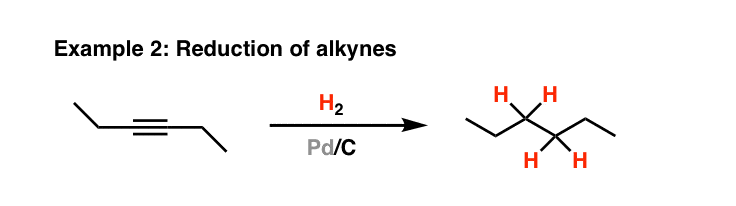

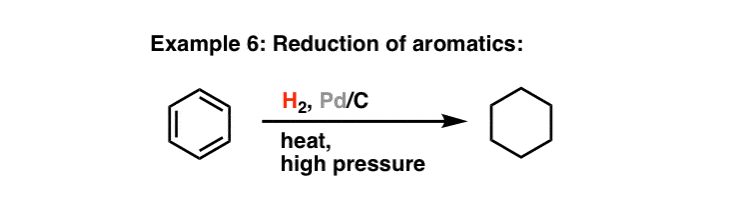

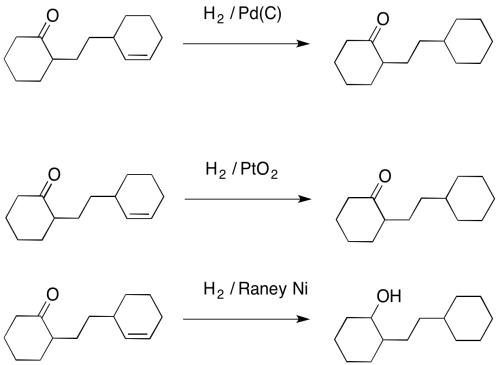

![Reductions of chalcones by ammonium formate/ palladium on carbon [12,13]. | Download Table Reductions of chalcones by ammonium formate/ palladium on carbon [12,13]. | Download Table](https://www.researchgate.net/profile/Wender_Silva/publication/233600118/figure/tbl1/AS:654364133830661@1533023975997/Reductions-of-chalcones-by-ammonium-formate-palladium-on-carbon-12-13.png)